The present structure of the atom is widely known. But how it got to where it is today is quite interesting. This journey includes many experiments and models. I’ll be going over them in chronological order and try to describe their history and contents as best as I can.
This post covers things related to Dalton’s theory, another posts will cover the next experiments and models.
Terminology
Atom : From the Greek
atomos(uncuttable), smallest unit of a chemical element.Element : The smallest possible unit of matter that cannot be decomposed into simpler substances by ordinary chemical processes.
Compound : The substance made from the combination of identical molecules of two or more elements in fixed proportion.
Molecule : Generally, a group of two or more atoms chemically bonded together. It’s also defined as ‘The smallest particle of a substance that is capable of an independent existence with all the properties of that substance’.
Atomic Mass : The mass of an atom relative to th of the mass of Carbon-12. That is, the masss of one atom of carbon-12.
Atomic Number : Number of protons found in the nucleus of an atom. Symbol (Z).
Mass Number : Number of total nucleons present in the nucleus of an atom, e.g., number of protons + neutrons.
John Dalton’s Atom
Dalton imagined atoms as sort billiard balls. As in — solid, uniform in shape and composition and indivisible in nature (though that maybe disproven on a billiard ball with enough force).
Dalton’s Atomic Theory.
Now these gradually became the core tenets of Dalton’s Atomic Theory.
1. Everything (elements) is composed of extremely small particles called atoms.
2. All atoms of a given element are identical in all respects & atoms of a different element are different in all respects (yup, different masses too).
3. Atoms are neither created nor destroyed in chemical reactions.
4. Compounds are formed when atoms of more than one element combine. A given compound always has the same relative number and kind of atoms.
5. Compounds always form when atoms combine in the ratio of simple whole numbers, e.g., 2:3 and 1:8 etc.
6. Atoms of elements may combine in different ratios to form different compounds.
7. An atom is the smallest particle that takes part in a chemical reaction.

An atom of hydrogen
Molecules and Compounds
Many elements have different types of molecules. Some elements like Argon (Ar)
have only one atom in their molecule and are thus, called monoatomic. Some
elements like Oxygen (O) have two atoms of oxygen in their molecule and are
called diatomic. The total number of atoms in the molecule of an element or
compound is called the atomicity of that element or compound.
The naming generally goes to tetra-atomic and to poly-atomic.
Compounds and Their Proportions
Atoms join together in definite proportions to form compounds. For example, water ( H2O ) has the mass ratio of 1:8. Since every water molecule has 4 hydrogen atoms for 2 oxygen atoms, the hydrogen atoms’ mass is and the oxygen atoms' mass is and . This means, no matter where you get your water from it’ll always have atoms of hydrogen by weight.
Laws of Chemical Reactions
Law of Conservation of Mass
This law was given by Antoine Lavoisier with experimental proof. This law states that the total mass of reactants will be equal to the total mass of the products, in a chemical reaction.
where Mass of (A + B) = Mass of (C + D).
Law of Constant Proportion
This law was also put forward by Lavoisier, it states that in a chemical substance the elements are always present in definite proportions by mass.
This was done because scientists at the time observed that all compounds would always have the same of amount of atoms by weight. For example, the mass ratio of hydrogen and oxygen was always found to be 1 : 8.
Valency
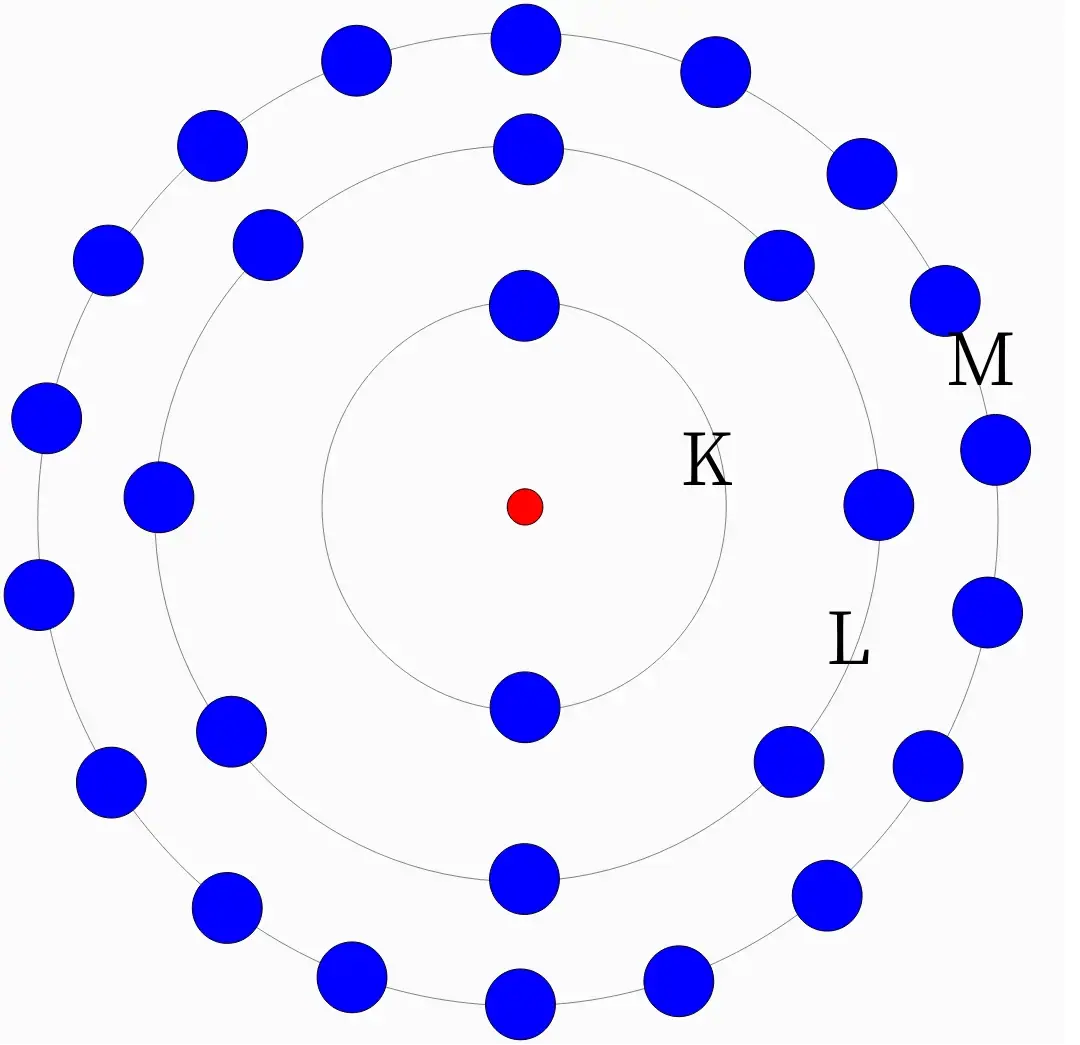
Every atom has multiple electron orbits around it. They are called orbitals K,
L, M and N. Each orbital is sometimes represented as n. So, if we’re
talking about K we’ll take n = 1, if we’re talking about L, we’ll take n = 2
and so on.
Now, since protons have a positive charge and electrons have a negative charge, they cancel out each other. Every atom that is neutral in charge has the name number of both protons and electrons. But how are these electrons arranged?
Each orbital (almost always) has amount of electrons. That is, if we're talking about orbital `M`, n = 3. Then, the no. of electrons in shell `M` will be .
Two chlorine atoms one electron away from an octet state
Octet State
Each atom seeks to have 8 electrons in its outermost shell, also called the
valence shell. However, this octet rule doesn’t apply to atoms that may lose
their outer electrons to accommodate the maximum number of electrons in their
next shell. For example, if helium has 2 electrons in it’s K shell then it’s
valency is 0 because this shell can only accommodate 2 electrons.
Similarly, hydrogen’s valency is 1 because it only needs one more electron to reach it’s limit of electrons (limit of 1) for it’s first shell. These won’t acquire more electrons in their other (non-existent) shells because their nucleus only has protons for that many electrons. That is, they don’t tend to acquire charge.
Now, atoms up to Boron work this way where they just lose their outermost electrons to keep maximum number of electrons in their immediate inner shell. After that atoms want to acquire electrons to have 8 electrons in their outermost shell.
As a general rule of thumb, if the number of electrons in the outermost shell is ≤ 3, the valency is just that number, that is, 3. If the number of electrons in the outermost shell is ≥ 4, then the valency is calculated the simple arithmetic of 8 - no. of electrons in outermost shell.
For example,
If the number of electrons in the outermost shell is is equal to or less than 3, say 2 electrons, then the valency will be 2.
If the number of electrons in the outermost shell is is equal to or more than 4, say 6 electrons, then the valency will be
The latter suggests that atoms may share their electrons with other compatible atoms such that both atoms can have 8 electrons in their outermost shell. And that’s completely correct! This is termed as ‘combing capacity’.
Therefore, an atom of each element has a definite combining capacity, called its valency.
Covalent Bonds
Now introducing bonds might be a bit too much for this one post but I will keep it brief. A chemical bond is an association of atoms to form molecule, compounds or other structures.
The bond made from the sharing of electrons between atoms in their outermost shell is called a covalent bond. I think that this fact, that atoms share electrons makes the concept of valency much more clear. And indeed this is why elements and compounds react with each other, to make covalent bonds and reach octet.

Two chlorine atoms one electron away from an octet state

Two chlorine atoms sharing one electron each to maintain their octet state